WASHINGTON — The US Food and Drug Administration (FDA) on Sept. 21 announced a proposed rule to establish additional traceability recordkeeping requirements for certain foods. The FDA also published the same day a draft food traceability list (FTL), which enumerated the specific foods that will be subject to the proposed requirements.
The FDA said the proposed rule, “Requirements for Additional Traceability Records for Certain Foods,” is a key component in the FDA’s New Era for Smarter Food Safety and would implement the section of the FDA Food Safety Modernization Act of 2010 (FSMA) that mandated the agency require more extensive product tracing records for foods the agency determined to be at “high risk” for contamination.
The Acheson Group noted the FTL foods were determined through FDA’s development of a risk-ranking model.
“Data was collected to populate the model for chemical and microbiological hazards associated with specific foods, and the draft risk-ranking model and data were reviewed by independent external experts,” Acheson said. “From this, FDA proposed 16 FTL foods, with the rule mandating additional traceability requirements for both the listed food and any food that contains a listed food as an ingredient.”
Foods on the draft FTL include sprouts, shell eggs, tomatoes, nut butter, tropical tree fruits, cucumbers, fresh-cut fruits and vegetables, fresh herbs, finfish, leafy greens (including fresh-cut leafy greens), crustaceans, melons, mollusks, peppers, and ready-to-eat salads.
The FDA said the proposal, if finalized, would standardize the data elements and information firms must establish and maintain, and the information they would need to send to the next entity in the supply chain to facilitate rapid and accurate traceability.
“While limited to only certain foods (those on the FTL), this proposal lays the foundation for a standardized approach to traceability recordkeeping, paving the way for the industry to adopt, harmonize, and leverage more digital traceability systems in the future,” the FDA said.
The FDA said existing regulations require much of the food industry to establish and maintain records to identify the immediate previous sources and the immediate subsequent recipients of foods.
“These requirements form a baseline for traceability recordkeeping, but they provide limited information to effectively and rapidly link shipments of food through each point in the supply chain,” the FDA said. “This — and the fact that recordkeeping systems can be largely paper-based and lack a universal lexicon throughout industry — can make it difficult to trace a product to its original source when necessary.”
The FDA said the improved traceability, as envisioned by the proposed rule, would allow the agency to more quickly identify the source of a contaminated product, reduce the scope of product recalls, and conduct more timely root-cause investigations to learn more about how contamination occurred in order to prevent future outbreaks.
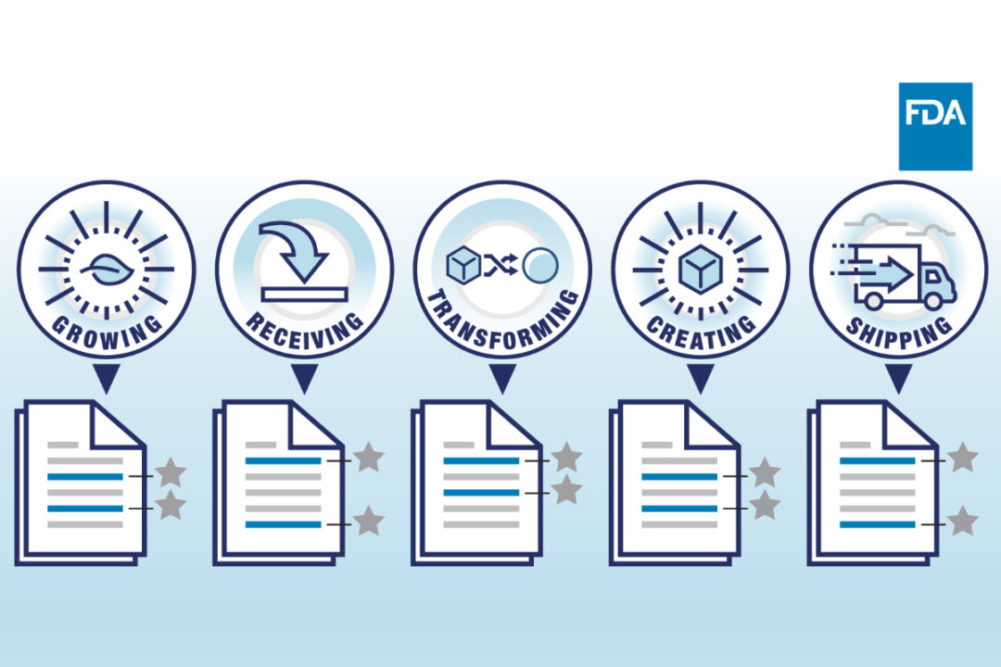
The FDA said at the heart of the proposal is a requirement for those who manufacture, process, pack, or hold a food on the FTL to establish and maintain records associated with specific critical tracking events, CTEs, which the FDA said comprised growing, receiving, transforming, creating, and shipping.
For each CTE, entities would be required to establish and maintain records containing key data elements, or KDEs.
The FDA said examples of KDEs include the traceability lot code, the date the product was received, the date the product was shipped, and a product description.
The FDA emphasized the traceability lot code is an important KDE throughout the supply chain intended to establish critical linkages that will help to facilitate rapid traceback and trace-forward investigations during foodborne illness outbreaks and recall events.
In addition, those subject to the rule also would be required to create and maintain records related to their internal traceability program, which would help regulators better understand a firm’s recordkeeping practices and traceability operations.
The proposed rule would require records to be maintained as either electronic, original paper records, or true copies. In addition, the proposal stated that in the event of a foodborne illness outbreak, a product recall, or other threat to public health, the FDA could require that firms submit, within 24 hours, an electronic sortable spreadsheet containing relevant traceability information for specific foods and date ranges.
The proposed rule includes several exemptions, including that the additional traceability records would not be required after a kill-step (a process that significantly minimizes pathogens in a food) is applied to a food, but documentation of the kill-step application would have to be established and maintained.
The proposed rule was published in the Federal Register on Sept. 23. The proposed rule and the draft FTL are available for public comment for 120 days from that date.
The FDA also will hold three public meetings on the proposed rule and FTL during the public comment period. The public meetings were scheduled for Nov. 6, Nov. 18 and Dec. 2.